How Changhong Yun Oligo LC-MS Revolutionizing Oligonucleotide Quantification
The analysis of oligonucleotides in biological samples is crucial for pharmacokinetic (PK), pharmacodynamic (PD), and toxicological evaluations during drug development. As oligonucleotide-based therapeutics become more prevalent, their unique distribution and metabolic profiles require advanced, sensitive analytical methods to ensure accurate quantification. The Changhong Yun Oligo LC-MS platform offers an effective solution for analyzing oligonucleotides and enhancing drug development processes by providing reliable data on drug stability, delivery efficiency, and metabolic pathways.
Ways changhong yun oligo lc-ms Revolutionizing Oligonucleotide Quantification
The LC-MS/MS method combines the high separation capabilities of liquid chromatography with the selectivity and sensitivity of mass spectrometry, making it ideal for the quantitative analysis of oligonucleotides. Unlike LBA and LC-FL, LC-MS/MS does not require the synthesis of specific probes, reducing the method's development time and cost. Moreover, this technique provides valuable information on the sequence structure and base composition of oligonucleotides, making it a powerful tool for metabolite identification and pharmacokinetic studies.
In addition to its ability to accurately quantify oligonucleotides in biological samples, LC-MS/MS facilitates the detection of low-concentration compounds, essential for drug development. Its use in early in vitro and in vivo screening provides quick, reliable, and sensitive data that accelerates the drug development process. Here is more detail about this:
Overcoming Challenges in LC-MS/MS
The analysis of oligonucleotides using LC-MS/MS comes with inherent challenges. Oligonucleotides are highly hydrophilic, exhibit low ionization efficiency, and often generate metal adduct ions, all of which can lead to poor peak shapes and low mass spectrometric responses.
To address challenges in analysis, three strategies are used. First, optimizing sample pretreatment (e.g., LLE and SPE) enhances oligonucleotide recovery by removing contaminants. Second, adjusting mass spectrometry parameters boosts ionization efficiency and sensitivity. Lastly, minimizing metal adduct formation by modifying chromatographic conditions improves peak resolution and response.
Sample Pretreatment and Method Development
Sample pretreatment is a critical step in LC-MS/MS analysis, impacting the extraction recovery and overall data quality. For oligonucleotide analysis, optimizing sample extraction methods is essential. Strategies such as using low-adsorption consumables, controlling pH during extraction, and employing nitrogen blow-dry techniques to concentrate samples ensure efficient oligonucleotide recovery from biological matrices.
Additionally, the development of suitable chromatographic conditions is crucial for the separation of oligonucleotides from interfering substances. Techniques like hydrophilic interaction liquid chromatography (HILIC) have shown promise in improving separation and minimizing ion suppression caused by traditional reverse-phase methods.
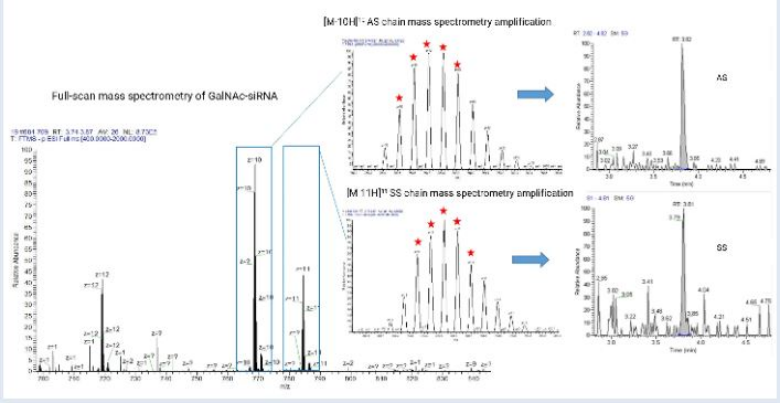
High-Resolution Mass Spectrometry (HRMS) in Oligonucleotide Analysis
The use of high-resolution mass spectrometry (HRMS), particularly Orbitrap HRMS, has become increasingly popular in oligonucleotide quantification. HRMS provides superior sensitivity, wide dynamic range, and high-resolution capabilities, enabling the detection of oligonucleotides at concentrations as low as 10 ng/mL in plasma. HRMS also offers advantages in metabolite identification through high-energy collision-induced dissociation (HDC), making it ideal for analyzing the pharmacokinetics and toxicology of oligonucleotides.

Furthermore, HRMS simplifies the optimization of MS parameters for various oligonucleotides, which can be challenging with traditional triple quadrupole MS. By providing full scan data, HRMS allows researchers to quantify oligonucleotides and identify impurities and metabolites using the same instrument.
Conclusion
The changhong yun oligo lc-ms platform represents a significant advancement in the field of oligonucleotide quantification. With its high sensitivity, rapid analysis, and ability to generate detailed structural information, LC-MS/MS is essential for pharmacokinetic and toxicokinetic studies of oligonucleotides.









3940